Anatomy of the testis
The human testis is an ovoid mass that lies within the scrotum. The average testicular volume is 20 cc in healthy young men and decreases in elderly men. In Asian men, testes tend to be smaller. Normal longitudinal length of the testis is approximately 4.5 to 5.1 cm. The testicular parenchyma is surrounded by a capsule containing blood vessels, smooth muscle fibers and nerve fibers sensitive to pressure. The functional role of the testicular capsule is unknown, but may relate to movement of fluid out through the rete testis or control of blood flow to the testis.
Testicular anatomy
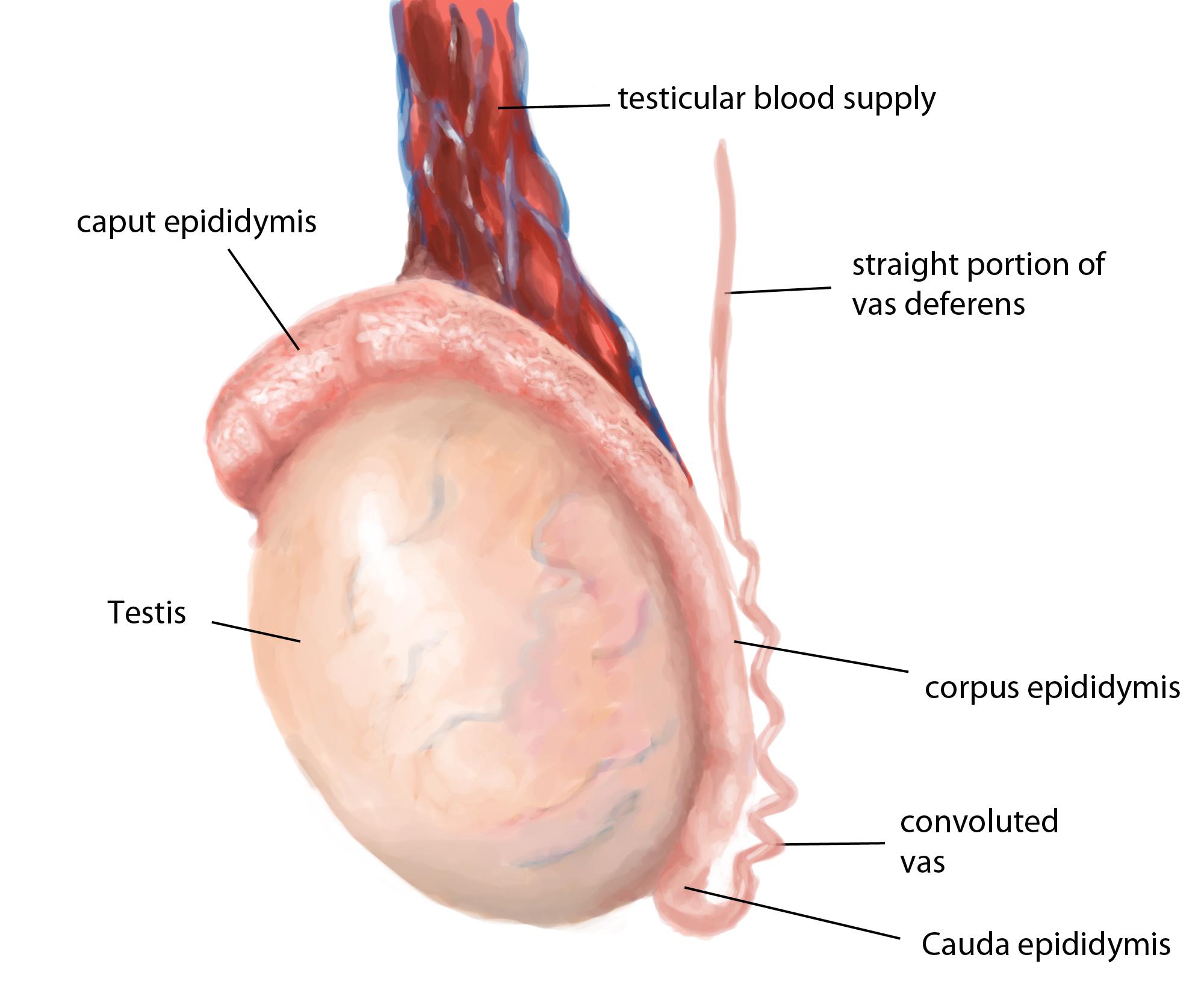
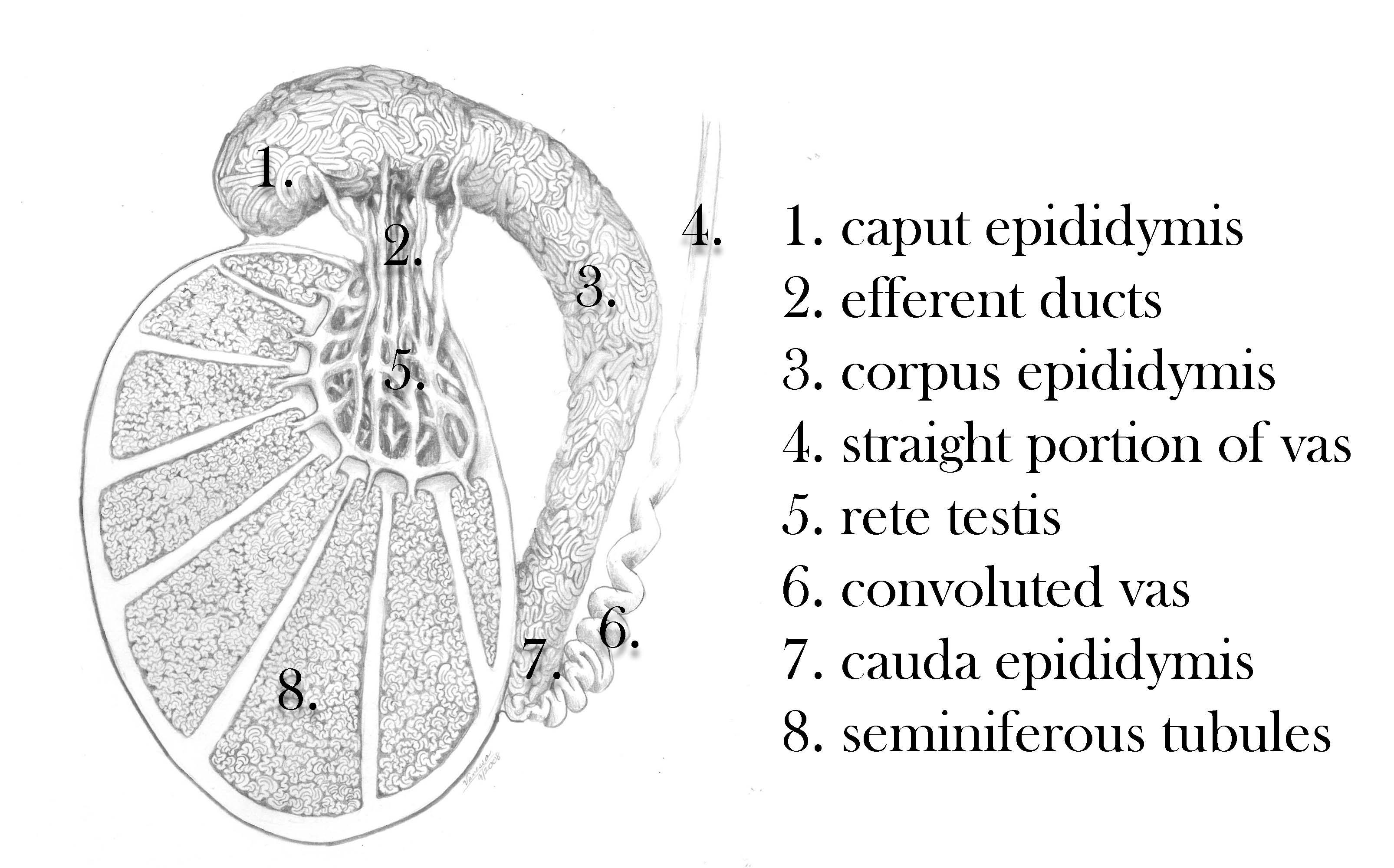
The testis contains seminiferous tubules and interstitial cells. The tubules are segregated into regions by connective tissue septa. The seminiferous tubules are long V-shaped tubules, both ends of which usually terminate in the rete testis. Measurement of testicular size is critical in the evaluation of the infertile man, since seminiferous tubules (the spermatogenetic region of the testis) occupy approximately 80% of testicular volume. So, a rough estimate of spermatogenic cell capacity is provided by assessment of testicular size. Testicular consistency is also of value in determining fertility capacity. A soft testis is likely to reflect degenerating or shrunken spermatogenic components within the seminiferous tubules. The seminiferous tubules drain toward the central superior and posterior regions of the testis, the rete testis, that has a flat cuboidal epithelium. The rete coalesces in the superior portion of the testis, just anterior to the testicular vessels, to form 5-10 efferent ductules. These efferent ducts leave the testis and travel a short distance to enter the head, or caput region of the epididymis. The efferent ducts coalesce in a somewhat variable pattern within the caput epididymis to form a single epididymal tubule.
The artery to the testis is specialized in that it is highly coiled and intimately associated with a network of anastomotic veins that form the pampiniform plexus. The counterflowing vessels are separated only by the thickness of their vascular wall in some areas. This vascular arrangement facilitates the exchange of heat and small molecules, including testosterone. The transport of testosterone is a concentration-limited, passive diffusion process in men. The counter-current exchange of heat in the spermatic cord provides blood to the testis that is 2 to 4 °C lower than rectal temperature in the normal individual. A loss of the temperature differential is associated with testicular dysfunction in humans with idiopathic infertility, as well as men with varicocele or cryptorchidism. Whether elevated testicular temperature causes or is simply a reflection of testicular dysfunction is unknown. Only the association between elevated testicular temperature and seminiferous failure have been demonstrated. In the distal inguinal canal, 50% of men will have a single testicular artery identifiable under l0 x power magnification dissection of the cord, with 30% of men having two arteries and 20% with three arteries.
The venous system is somewhat unique because the spermatic veins are thin-walled, poorly muscularized, and lack effective valves except at the inflow points into the inferior vena cava or the renal vein. The right spermatic vein usually drains into the vena cava. The left spermatic vein drains into the left renal vein. The renal vein on the left side is thought to have a higher intraluminal pressure because the vein is compressed as it passes between the superior mesenteric artery and the aorta. This "nutcracker effect" may impair flow through the left renal and spermatic veins, especially in young men with limited retroperitoneal fat. The differential anatomy of the left and right spermatic veins is thought to explain, at least in part, the higher prevalence of varicoceles on the left side. The exact mechanism by which varicoceles cause infertility is unknown. In animal models, varicoceles are associated with increased blood flow to the testis and increased interstitial fluid in the testis. These two findings may impair regulation of testicular temperature and decrease intratesticular concentrations of testosterone or other local factors important for spermatogenesis.

